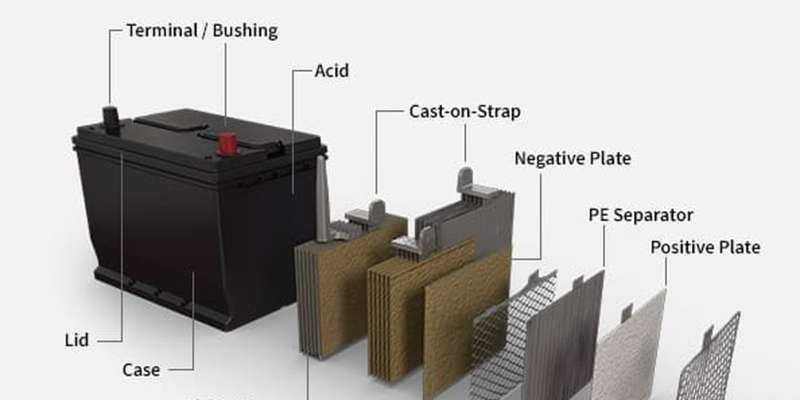
Car batteries are the unsung heroes of our vehicles. They are more than just a box under the hood; they are the heart of your car’s electrical system, providing the necessary power to start the engine and run all the electrical components. Whether you drive a traditional gasoline-powered car or an electric vehicle, a functioning battery is absolutely essential to get you on the road. To understand how to maintain your car and troubleshoot issues, it’s helpful to know what’s inside this vital component. This article will serve as your comprehensive Car Battery Parts List, detailing each component and its function.
What is a Car Battery?
At its core, a car battery is a device that converts chemical energy into electrical energy. This electrochemical reaction is what provides the initial burst of power needed to start your engine and keeps your car’s electrical systems running. The vast majority of car batteries are rechargeable, designed as wet-cell batteries, and they are crucial for powering everything from the ignition system to your headlights and radio.
When you turn the ignition key, the car battery immediately springs into action, delivering an electric current that sets off the internal combustion process in gasoline engines or powers the electric motors in EVs. Beyond starting the car, the battery ensures a consistent power supply for all other electronic components, including lights, infotainment systems, windshield wipers, and more, ensuring a safe and comfortable driving experience.
Car Battery Parts List: Key Components Explained
A typical car battery is housed within a protective case, but the real magic happens inside. Let’s break down the essential car battery parts list and understand what each component does:
Battery Case
The battery case, or housing, is the robust outer shell that encases all the internal components of the car battery. This case is critical for protection, shielding the delicate internal parts from physical damage, vibrations from the vehicle, and extreme temperatures. For traditional lead-acid batteries commonly found in fuel-based vehicles, the cases are often constructed from durable polypropylene resins. In electric vehicles, where battery packs can be significantly larger and more complex, the enclosures often utilize lightweight yet strong aluminum alloys. The battery case is designed to extend the battery’s lifespan by minimizing external impacts and containing the battery’s internal components securely.
Battery Acid (Electrolyte)
Often referred to as battery acid, the electrolyte is a solution of sulfuric acid and water, typically ranging from 36% to 40% concentration. This solution plays a crucial role in the battery’s operation as the electrolyte that facilitates the chemical reactions necessary to produce electrical current. The purity of this acid is paramount, as contaminants can negatively affect its efficiency in generating power.
When the car is started, the battery acid reacts chemically with other components inside the battery, generating the required voltage to initiate the engine. A weak or depleted battery often struggles to produce sufficient voltage, leading to starting problems.
Battery Terminals
Battery terminals serve as the vital connection points that link the car battery to the vehicle’s electrical system. These terminals, sometimes called battery bushings, are designed to securely and efficiently conduct electricity. Every car battery has two terminals: a positive terminal (marked with a + symbol) and a negative terminal (marked with a – symbol). These terminals are usually made of lead and are specifically designed to accommodate battery cables, ensuring a solid and reliable electrical connection.
Battery Plates
Inside each car battery are multiple plates, categorized as positive and negative plates. Each plate consists of a metallic grid structure. The positive plates are coated with lead dioxide, while the negative plates are made of spongy lead. These materials are specifically chosen for their electrochemical properties, which are essential for the battery’s charge and discharge cycles. At the top of each set of plates is a cast-on strap, a conductive element that connects each plate to the battery cells, ensuring efficient current flow throughout the battery.
Battery Separator
The battery separator is a crucial, yet often unseen, component within a car battery. Its primary function is to electrically isolate the positive and negative plates from each other, preventing them from making direct contact and causing a short circuit. Despite providing electrical insulation, the separator is designed to be porous, allowing the electrolyte to freely flow between the plates. Separators are typically made from materials like polyethylene or other specialized plastic polymers that are resistant to the battery acid and can withstand the internal battery environment.
Working Principles of Car Batteries
The main job of a car battery is to supply the electrical energy needed to power all of a vehicle’s electrical systems. Even when your car engine is off, the battery continues to provide power to certain components like the clock, alarm system, and in some cases, even the car radio. However, the high power demand for driving the car engine is only required upon ignition.
Here’s a simplified breakdown of how a car battery works when you start your car:
- Chemical Reaction: When you turn the key, a chemical reaction is initiated within the battery. This process converts the stored chemical energy into electrical energy.
- Voltage Delivery: This electrical energy is then released, providing the necessary voltage to the car’s starter motor and other electrical systems.
- Voltage Regulation: The battery also plays a stabilizing role, ensuring a consistent flow of electrical current by regulating the voltage. This regulation is crucial because unregulated voltage fluctuations could damage sensitive electrical components in your vehicle.
It’s important to note that starting a car engine typically only requires a small percentage of the battery’s total capacity, often around three percent. Car batteries are designed to deliver a high current burst for a short duration, primarily to start the engine and power the ignition and lighting systems. This is why they are sometimes referred to as SLI batteries – Standing, Lighting, and Ignition batteries – highlighting their core functions.
Different Types of Auto Batteries
The type of car battery in your vehicle can significantly impact its performance and longevity. Choosing the right type of battery is essential for optimal vehicle operation. Here’s a brief overview of different car battery types:
Primary Cell Batteries
Primary cell batteries are designed for single use. Common examples include standard alkaline batteries like AA or AAA batteries used in household devices like remote controls. These are non-rechargeable and are not typically used in automobiles due to their inability to be recharged. Their use is declining overall as they must be discarded after their charge is depleted.
Secondary Cell Batteries
Secondary cell batteries, in contrast to primary batteries, are rechargeable, making them suitable for long-term use in vehicles. They contain electrolytic materials—the electrolyte and electrodes—that enable them to be repeatedly charged and discharged. These are the standard battery type for cars, with the most common types being:
- Lead-Acid Batteries: These are the most traditional type of rechargeable car battery and are widely used in gasoline-powered vehicles. Lead-acid batteries were pivotal in the development of rechargeable battery technology. They are known for providing a high power output relative to their size and weight, making them not only useful in cars but also as backup power sources in settings like hospitals and telecommunication towers.
- Lithium-Ion (Li-ion) Batteries: Lithium-ion batteries are increasingly common in modern vehicles, particularly in electric vehicles and hybrids. They offer a high energy density, meaning they can store a significant amount of energy in a relatively lightweight package. This high energy capacity allows EVs to achieve longer driving ranges on a single charge. Li-ion batteries also have a low self-discharge rate, meaning they retain their charge well even when not in use for extended periods.
Solid-State Batteries
Solid-state batteries represent a newer advancement in battery technology. They are designed to eliminate the need for a liquid electrolyte, which is characteristic of traditional batteries. Instead, they use solid, ceramic-like materials as the electrolyte. This innovation offers several potential advantages, including faster charging times, improved safety, and potentially higher energy density. Solid-state batteries are becoming increasingly relevant in the electric vehicle sector due to these enhanced capabilities.
Functions of Car Batteries
Car batteries perform several critical functions beyond simply starting the engine. Here’s a summary of the key roles they play in your vehicle:
Engine Starter
Perhaps the most crucial function of a car battery is to start the engine. The battery acts as the primary power source, converting chemical energy into electrical energy upon ignition. This electrical energy is then distributed to the starter motor and ignition system, enabling the engine to turn over and start running. Without a functional battery, starting a conventional car engine is virtually impossible.
Power Storage
The car battery stores the electrical energy needed not only for starting the engine but also for powering various electrical accessories when the engine is not running. A good quality battery should be capable of retaining its charge over time and reliably power the vehicle when properly connected, even after periods of storage. Once the engine is running, the alternator takes over most of the electrical power supply and also recharges the battery, ensuring it’s ready for the next start and has sufficient power to support all electrical systems.
Collaboration With Alternator to Power Other Electrical Components
While the alternator supplies power when the engine is running, the battery works in conjunction with the alternator to power the vehicle’s electrical components. The alternator is responsible for continuously generating electricity while the engine is running, which powers systems like the headlights, air conditioning, windshield wipers, and infotainment system. The battery supplements the alternator, especially when the electrical demand is high or when the engine is idling and the alternator output is lower.
Regulates Voltage
Modern car batteries are designed to help regulate the voltage within the vehicle’s electrical system. Sometimes, other components can cause voltage spikes or fluctuations, which can be harmful to the car’s sensitive electronics. The battery can act as a buffer, absorbing excess energy and stabilizing the voltage, thus protecting the vehicle’s electrical system from potential damage due to voltage irregularities.
Conclusion
Understanding the car battery parts list and their functions is crucial for any car owner. A well-maintained car battery is essential for reliable vehicle operation. If any of the car battery parts are damaged or malfunctioning, the battery’s ability to power your vehicle can be compromised. The robust battery case ensures that all these critical components remain protected and function effectively, contributing to the overall reliability of your vehicle.
FAQs
What are the basic components of a car battery?
The fundamental components of a car battery include the electrolyte (battery acid or lithium salt solution), positive and negative plates (anode and cathode), separators, terminals, and the battery case. The case is vital for containment and protection, ensuring safety and preventing leaks of the potentially hazardous materials inside.
What is the liquid inside a car battery?
In a traditional lead-acid car battery, the liquid is the electrolyte, often called battery acid, which is a solution of sulfuric acid and water. In lithium-ion batteries, the liquid electrolyte is a solution of lithium salt dissolved in organic solvents, such as LiPF6, LiClO4, or LiBF4, depending on the battery chemistry.